
Researchers Ankur Singh, John Blazeck, Fatih Sarioglu, and Gabe Kwong. Not pictured: Susan Thomas. (Photo: Candler Hobbs)
(text and background only visible when logged in)
Engineers are rewiring cells and creating new tools to improve cancer therapies and catch the disease earlier.
(text and background only visible when logged in)
By now, we were supposed to be able to treat just about every kind of cancer.
President Richard Nixon declared war on the disease in 1971 during his State of the Union speech, pledging to ask Congress for $100 million to find a cure for what was then the second-leading cause of death in America.
“It was thought that we would be able to solve it in about a decade; that was the goal. And we’ve learned over time, cancer is actually much more complex than we gave it credit for in the beginning,” said Gabe Kwong, a Georgia Tech biomedical engineer working toward the goal Nixon laid out more than 50 years ago.
“There are many subtypes of cancer, and each requires a different strategy. And yet, as a population, we’re living longer and longer. People are developing cancers because it’s a disease of aging, basically. So, it’s a big problem, it’s very complex, and it requires new ways of thinking about it.”
Kwong is an associate professor in the Wallace H. Coulter Department of Biomedical Engineering, a joint program shared equally by Georgia Tech and Emory University. For all the reasons he outlined, cancer is a challenging problem.
And who is drawn to big, challenging problems more than engineers?
Kwong’s efforts got a significant boost this fall when President Joe Biden’s Cancer Moonshot Initiative awarded up to $50 million to support a project Kwong has been building to for more than a decade.
He’s assembled a team to develop a new generation of tests capable of detecting multiple types of cancers earlier than ever, allowing doctors to start treatment when tumors are still small and most responsive.
The key is what Kwong calls the Cancer and Organ Degradome Atlas, or CODA platform, which will map the unique cellular profiles of cancer cells and leverage that knowledge to build new bioengineered sensors to detect those profiles.
An Atlas for Finding Cancer Earlier
Biomedical engineer Gabe Kwong is leading a $50M Cancer Moonshot project to map cancer cell biomarkers. The goal is a new test capable of finding multiple types of cancer earlier than ever.
Early detection is possible now, but only for a handful of cancers. Kwong’s vision is to build a one-size-fits-all product that could do the work of those existing tests — mammograms, colonoscopies, and Pap smears, for example — plus detect cancer types currently without reliable tests for early detection.
“This is what researchers like to do — we dream about a different future; about the technologies we need to develop to get there,” Kwong said. “I’ve been cultivating this vision for the last 10 years. Now there’s a mechanism to implement it and go at light speed. That’s pretty exciting.”
It’s hardly the only exciting work happening in the College of Engineering to make untreatable cancers treatable and improve the lives of patients.
Some researchers are using the body’s own immune system to counteract and combat cancer, including significant efforts to leverage the capabilities of T cells — perhaps the body’s most powerful cells. They’re creating new ways to study, or change, the environment around tumors. And they’re also building new tools to help demystify the “heterogeneity” of cancers, including why some cancers — like those of the prostate — take a far greater toll on Black and African Americans.
(text and background only visible when logged in)
Cancer ‘Microchips’
Answering at least one part of that question is the subject of an ongoing multi-year study involving Georgia Tech electrical engineers and researchers at Emory University’s Winship Cancer Institute.
Black men are twice as likely to die from prostate cancer and nearly twice as likely to be diagnosed in the first place, according to the American Cancer Society. Fatih Sarioglu and his collaborators have been looking at tumor cells in blood samples from Black prostate cancer patients to isolate whether biological or physiological differences can account for those numbers.
It’s a project made possible by technology Sarioglu’s lab has developed that can pluck a single circulating tumor cell out of the 50 billion or so cells in a test tube of blood.
“It’s still an evolving area, because the challenge has always been to reliably and robustly get these circulating tumor cells,” said Sarioglu, associate professor in the School of Electrical and Computer Engineering. “Only when you have reliable technology, when you make them studiable, can the research and the clinical applications start.”
Using the same methods used to build computer chips, Sarioglu has developed microfluidic chips with tiny, cell-sized channels or pores that blood samples flow through. Along the way, tumor cells are caught for study.

ECE Professor Fatih Sarioglu, left, and researchers work on a 3D-printed cell trap that captures tumor cells from diagnostic blood samples. (Photo: Allison Carter)
In one version, his team used a 3D printer to create a two-step approach: The chip first captured large white blood cells using common proteins on their surface and then used a filter that allowed much smaller red blood cells and platelets through but stopped larger tumor cells. Another approach, called Cluster-Wells, filtered blood to capture tumor cells that have clustered together. They’re more efficient at spreading cancer, Sarioglu said, but also have an interesting biophysical marker — the clumping — that allows him to find them in blood.
“We can design systems that will make sure every cell in that blood sample will go through the same testing. And then you can design a chip that will ‘touch’ essentially each of these cells, pick the ones that are tumor cells, and let the other cells go away,” Sarioglu said. “That way you can actually find a needle in a haystack.”

Other approaches exist to capture tumor cells in the blood, but they’re typically too aggressive and damage the cells in the process or break up clusters. Sarioglu’s techniques are both effective and sensitive, allowing clinicians to study tumor cell proteins or do full genome sequencing just like they would with tumors themselves.
Catching circulating cells sooner improves the chances of stopping the tumor’s spread, a powerful tool to fight metastatic cancer, which is responsible for most cancer deaths.
“Most of the time with a solid tumor, you have these cells circulating in the bloodstream. People have known this for more than 100 years,” Sarioglu said. “People knew that the cells were killing the patient, but we didn’t have a way of getting them.”
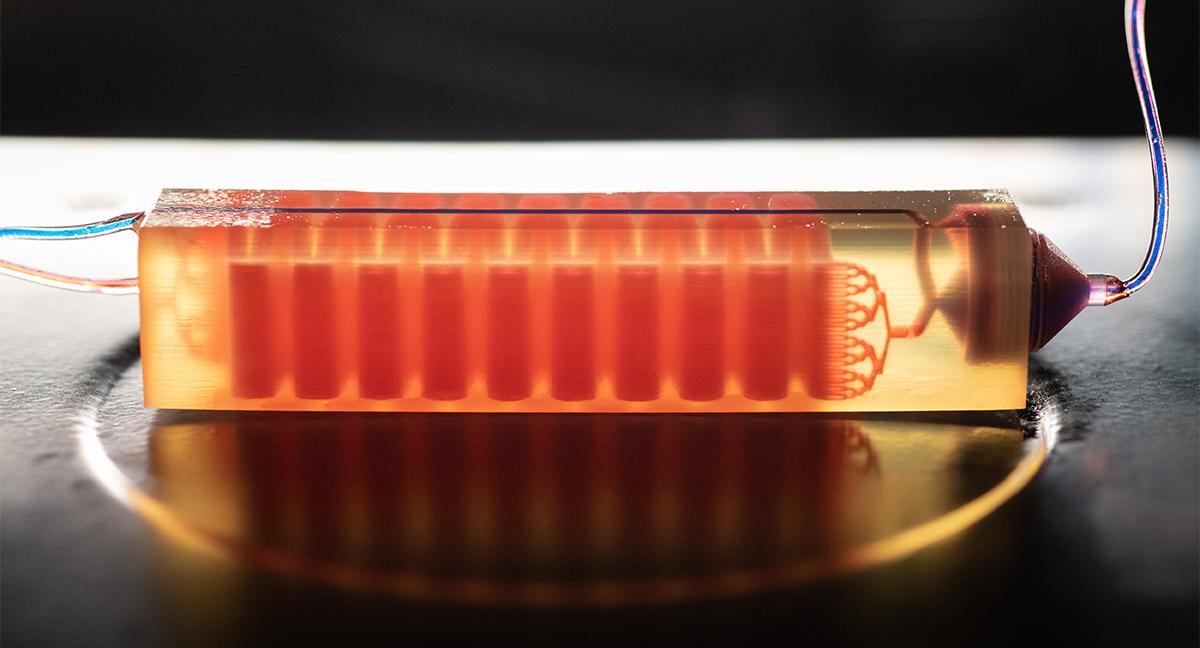
Now they do, and the chips his team is designing also can be used for other biofluids where cancer cells might be present. Blood is incredibly complex, but it’s easy to acquire noninvasively, so it’s the natural starting point.
One day, the screening tests could be part of everyone’s annual checkup, Sarioglu said, just to check for early signs of cancer. His chips don’t degrade the blood samples — they’re not diluted and nothing is added — so the typical panel of blood tests could still happen after the sample passes through.
Most immediately, though, Sarioglu’s team will continue processing blood samples in his collaboration with Emory in hopes of finding a thread that will begin to unravel why prostate cancer is so much more deadly for Black patients.
“This aggressive behavior of the disease has been known for a long time, but now we are really well positioned at Georgia Tech to do this research,” he said. “We have excellent clinical collaborators at Emory, we have a large African American population in this area, and then we have these capabilities on campus — I think everything is coming together and this is the right time to go after this kind of problem.”
(text and background only visible when logged in)
New Chip Could Make Treating Metastatic Cancer Easier and Faster
Sarioglu’s lab has found a detection method that could revolutionize cancer treatment by showing how cancers metastasize and what stage they are.
A Question of Environment
Ankur Singh’s immunoengineering lab is tackling disease disparities for a different cancer. Lymphoma — cancer of the lymphatic system — affects people of all races and ethnicities but occurs in Black patients at a younger age than other groups, and the disease is often more advanced. Black patients also don’t respond to treatment as well as white patients.

Singh in his lab with a synthetic hydrogel his team developed to grow organoids to study prostate cancer. (Photo: Jerry Grillo)
Working with Emory University oncologist Jean Koff, Singh hopes to unravel how the area around tumors in the body — the tumor microenvironment — plays a role.
“There is evidence that the mutational landscape is different for those patients across demographics in terms of the tumor cells, but what about the tumor microenvironment? There is absolutely no solid information out there,” said Singh, professor in the Coulter Department and the George W. Woodruff School of Mechanical Engineering.
“There’s reason to believe all of this is happening because of a combination effect of mutation and microenvironment. We understand the mutation through genetic studies; we do not understand the impact of microenvironment. And that’s where my lab is leading.”
Singh is fascinated by the complex interplay of cancerous growths and other cells nearby. Those interactions impact how the cancer progresses, or doesn’t, and why treatments work for some patients yet fail for others.
To understand those interactions, Singh’s research group creates models of tumors and lymph tissues in the lab using materials called hydrogels and uses chip-based models to study the intricate movement of fluid in tissues.
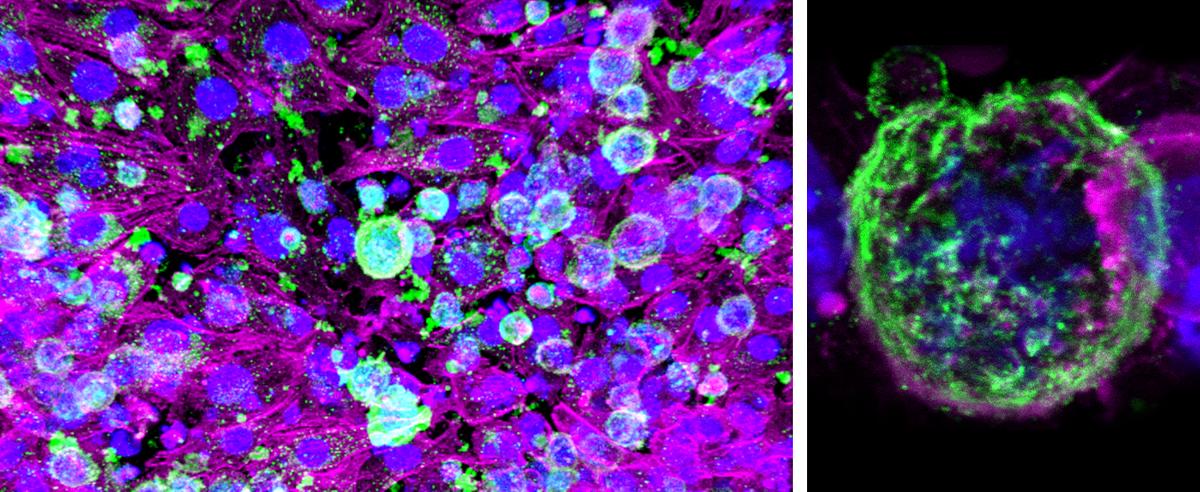
A model of a lymphoma tumor called an organoid that allows Ankur Singh to study the complex interactions between cancerous growths and the cells nearby. Green represents protein markers for tumor cells, and magenta areas are protein markers for supporting connective tissue cells. (Photos courtesy: Ankur Singh)
Earlier this year, his team published details of a new synthetic hydrogel-based model of the lymphoma microenvironment. They used data from more than 1,100 patients with a specific kind of lymphoma to create their platform, and they were able to grow tumors from patient samples. Singh’s team discovered how the tumors were hijacking the immune system to resist a new kind of inhibitor drug, even at high doses.
“We demonstrated that there is a massive dampening of therapeutic response when you have certain kinds of immune cells present in the tumor microenvironment,” he said. “It is only by using a precision approach of combination therapy that you can overcome those tumors.”
That, he said, is the power of seeing the whole picture: tumor plus its environment. Ultimately, he’s working toward models that could be adjusted to fit every specific tumor and patient to help doctors try treatments before they give them to patients.
“Our approach is that every patient’s tumor is different. Every patient is different. Black patients versus white patients, male versus female, young versus old, obese versus lean, all of those factors are tied into it,” Singh said. “We’re working to make a technology where you can plug-and-play and change the components to meet the needs of that particular patient’s tumor.”
(text and background only visible when logged in)
Therapeutic Superhighway
Immunoengineer Susan Thomas also spends her days thinking about lymph nodes.
Thomas specializes in drug delivery — getting therapies to exactly the place they’re needed. And she sees the lymphatic system as the superhighway those therapies can travel.
“When you get vaccinated or have a sore throat, your lymph nodes swell. They’re essentially tissues where tons of immune cells in your body gather. Our thought is to help the magic happen where all the cells are,” said Thomas, professor in the Woodruff School.
Her lab creates biomaterial drug delivery technologies that capitalize on those gatherings. Getting drugs into the lymphatic system means they can reach niches where immune cells gather in high numbers before they distribute elsewhere to fight disease.
“The idea is you want to activate the immune system to go and survey your entire body looking for cancers and then try to kill it,” Thomas said. “And that works for many types of cancer.”
One powerful cancer therapy is a relatively new type of drug called an immune checkpoint inhibitor, which counteracts a trick that tumors play on the body’s immune system. Normally T cells would identify the foreign tumor cells and destroy them. But some tumors produce immune checkpoint proteins, so the T cells think the tumor cells are part of the body and leave them alone.

Immune checkpoint inhibitors essentially block these checkpoint proteins, preventing the tumor from turning off the T cells. Often, though, these therapies work only for a portion of patients. Thomas’ work aims to help those immune checkpoint blockade drugs work better and at a lower overall dose.
“By micro-dosing lymph nodes with those immune checkpoint blockade drugs, you’re getting some of that drug to many cells in one spot. Those cells then leave that lymph node and travel elsewhere in the body. They are now more capable of killing cancer cells, and this was achieved using the drug at a very low dose systemically,” she said.
Traditionally, the approach has been to try to deliver cancer drugs throughout the body or directly to the tumor, she said. “What we’re doing is fundamentally different. We’re trying to get the drug to the immune cell wherever it is, and we think a good place to look is where they naturally congregate — lymph nodes. After interacting with the drug, the immune cell is changed and behaves differently once it is in the tumor. We don’t need the drug to be in the tumor to still have the effects. And we think that is part of the reason why our systems are working so well, because it’s getting more cells to behave in that beneficial, different way.”
Another project is a collaboration with the Woodruff School’s Brandon Dixon to improve the quality of life for patients after cancer treatment.
About a fourth of breast cancer patients experience swollen limbs after a mastectomy or lumpectomy that also removes lymph nodes. This prevents fluid from draining out of tissues properly, and the resulting lymphedema is uncomfortable and irreversible. Thomas and Dixon created a nanoparticle that delivers a drug directly to failing lymph vessels, restoring their ability to pump fluid and drain tissues.
Since they published a study on their nanoparticle, Thomas has received emails and phone calls almost every day from patients with lymphedema looking for relief and hoping the team’s work will lead to a trial they can participate in.
“They’ve survived their cancer, but they don’t know what to do about the lymphedema,” Thomas said. “We always think about trying to save patients. We need to also start thinking more about quality of life for patients in the wake of their treatment.”
Nanotechnology Approach Shows Promise for Treating Lymphedema
ME’s Brandon Dixon and Susan Thomas use nanoparticles to deliver a drug therapy directly to lymph vessels and restore their fluid-draining function.
(text and background only visible when logged in)
Metabolic Machinery

John Blazeck (Photo: Candler Hobbs)
The immune system’s T cells are the focus for chemical engineer John Blazeck, too. And like Singh, he’s looking less at tumors themselves and more at their environment.
Blazeck studies changes in metabolism that occur in and around solid tumors, a hallmark of many types of cancer. He’s thinking about ways to make that environment less favorable so cutting-edge immunotherapies are more effective for more people. He’s particularly interested in lung and breast cancers.
“Those are the ones that we study the most, in part, because the metabolic changes that occur seem to really impact the efficacy of current frontline immunotherapies,” said Blazeck, assistant professor in the School of Chemical and Biomolecular Engineering. “This is where our contributions could have direct relevance in helping the current best-in-class treatments.”
Blazeck has received significant funding from the National Institutes of Health (NIH) to explore two parallel approaches to make the tumor’s environment less hospitable.
Often tumors use up the nutrients around them faster than they can be replenished — think energy molecules like glucose and protein building blocks like amino acids. The tumors might be growing faster than other cells and have less blood supply, so they vacuum up nearby nutrients, simultaneously depriving the body’s immune cells of tools they need to grow fast and kill the tumor cells.
“We want to rewire the metabolism of T cells, enhancing it so that they can outcompete tumor cells for some of these limited nutrients in the tumor environment and resist this nutrient depletion,” Blazeck said.
That’s just part of the one-two punch tumors deliver to the body’s metabolism. They also overproduce metabolites, leftovers or byproducts of metabolism. The accumulation of these molecules interferes with T cells, basically turning them off.
Blazeck is working to engineer enzymes to degrade these immunosuppressive metabolites, freeing the T cells to attack the growing tumor.
Right now, he’s exploring both paths in parallel. But the hope is they could eventually converge into a combination approach.
“Our idea to use cells to remodel their metabolic environment hasn’t really been attempted,” Blazeck said. “If we succeed, it would be the first potential way to use an engineered cell therapy to change the extracellular metabolic environment of the cell itself.”
(text and background only visible when logged in)
Living Sensors
Blazeck also is part of the multi-institutional research team Gabe Kwong has assembled for his $50 million Cancer Moonshot effort to build an atlas of cancer cell biomarkers and create tests that can detect multiple cancers early in their development.
Kwong has long used cell engineering techniques to create cell therapies or diagnostics. He’s co-founded two biotech companies working to commercialize some of the innovations his team has built in the lab.

Gabe Kwong (standing) and Ph.D. student Ali Zamat added a genetic on-off switch to CAR T cells and developed a heat-activation system to enhance how the cells attack tumors. (Photo: Jerry Grillo)
In one line of inquiry, Kwong is turning the immune system’s T cells into mini drug factories right at the tumor site.
It’s an extension of chimeric antigen receptor (CAR) T cell therapy. The idea is to engineer the T cells to produce powerful but toxic drugs, and then activate them with a focused ultrasound beam.
“We’re using that technology to give us spatial precision so we can turn on these T cells and locally produce a drug that you would not dare to use systemically,” Kwong said.
Over the last year or so, he’s also been working on a different project to turn immune cells into roving sentinels watching for tumor growth.
For more than a decade, Kwong has developed synthetic biosensors that can tell doctors if a patient is responding to immune checkpoint blockade therapies or watch for metastasizing cancers. Those sensors are like inorganic chemical probes that produce a signal in urine or blood. Kwong said engineers can do a lot of things with those systems, but the limitations are real. They don’t proliferate and they don’t have memory, for example.
Some immune cells do; they patrol the whole body, they can destroy target cells, and some remember once they’ve seen a molecule or antigen. That makes them an exciting potential tool to watch for, produce an alert, and even eradicate cancer lesions early, he said.
“Immune cells have already evolved all these functions. We, as engineers, just need to know how to harness them and redirect — and in some cases, rewire — these functions to do the things that we want the cell to do.”
Kwong’s team has created the initial proof of concept of that rewiring in the lab, engineering immune cells with tumor-sensing receptors and programming them to secrete a molecule that can be detected when they find tumor cells.
Kwong said the idea of engineering immune cells as sensors will help lead us to a new era of preventative oncology. He could see his engineered cells even being used to monitor patients who are high risk but don’t have cancer.
“We could imagine a future where this is safe and inexpensive enough that you do this in healthy individuals. You pre-engineer a small subset of their immune cells to report on the cancer that they’re susceptible to,” Kwong said. “That’s where we’re trying to go. That vision will take maybe 10 or even 15 years, but that’s a real possibility in my view.”

(text and background only visible when logged in)
Integrated Cancer Research Center
Cancer researchers at Georgia Tech created a research consortium focused on harnessing expertise across disciplines to develop new diagnostics and therapeutic approaches for cancer. The Integrated Cancer Research Center aims to build on Tech’s history of collaboration in those areas, coordinating efforts across campus and with Tech’s partner institutions.
(text and background only visible when logged in)
Helping Families Keep a Watchful Eye
Living biosensors constantly patrolling for cancers would be a game-changer for families who are genetically predisposed to get certain types of the disease. In the meantime, researchers at Georgia Tech, Emory, and the Georgia Clinical & Translational Science Alliance (Georgia CTSA) are working to help these families with a very practical need: keeping track of their intense schedule of tests and doctor appointments.
Roughly 5% to 10% of cancer malignancies occur in patients with cancer predispositions. For these patients and their families, continual surveillance is a fact of life to catch cancer early, when it is most treatable. That means regular blood work, MRIs or other imaging scans, and other procedures.
It can quickly become overwhelming: Because the predisposition is genetic, that usually means everyone in the family is on some kind of testing regimen.
“Keeping track of these appointments is important. We know patients who miss their surveillance studies are worse off from a clinical perspective, and they have a worse prognosis,” said Wilbur Lam, professor in the Coulter Department of Biomedical Engineering and in the Emory University Department of Pediatrics.
Lam leads the innovation catalyst at Georgia CTSA, including AppHatchery, which pulled together clinicians, engineers, and software developers to create a mobile app to help these families avoid the health impacts of missing their regular scans. Called HomeTown, their app is “a simple but elegant solution to addressing a very important clinical problem for these patients,” Lam said.
Based on conversations with families, the app includes a calendar to track appointments and recommended tests for each family member along with reminders to schedule those tests. Users can store test results and notes from doctor’s appointments and research recommended surveillance protocols. In its initial form, the app includes guidelines for six of the most common cancer predispositions.
“HomeTown can help streamline complex care for patients and families who have a cancer predisposition syndrome. For example, the reminder feature helps them keep track of scheduling various cancer surveillance interventions. This can be extremely cumbersome, especially when one caregiver is coordinating surveillance for a number of family members or children,” said Sarah Mitchell, a physician at Children’s Healthcare of Atlanta who collaborated on the app.
Beyond keeping up with critical monitoring regimes, the development team also found the app helped reduce the “mental load” that comes with the constant worry about what test results will show, according to lead developer Santiago Arconada Alvarez.
“The period between the scan and the result is really stressful,” said Arconada Alvarez, scientific director of the Georgia CTSA AppHatchery. “Anything we can do to offload what’s going on in their head, to help them trust that it’s in the app and they don’t also have to carry it as part of their daily lives, we found to be helpful to these caregivers and families.”
In the initial testing, more than 150 families around the country downloaded and used the app. The team is hoping for continued growth.
“I can definitely see an app like this being incorporated into patients’ lives,” Mitchell said. “Everyone seems to have a smartphone, and a mobile app like this could be an incredible resource for patients, families, and healthcare providers.”
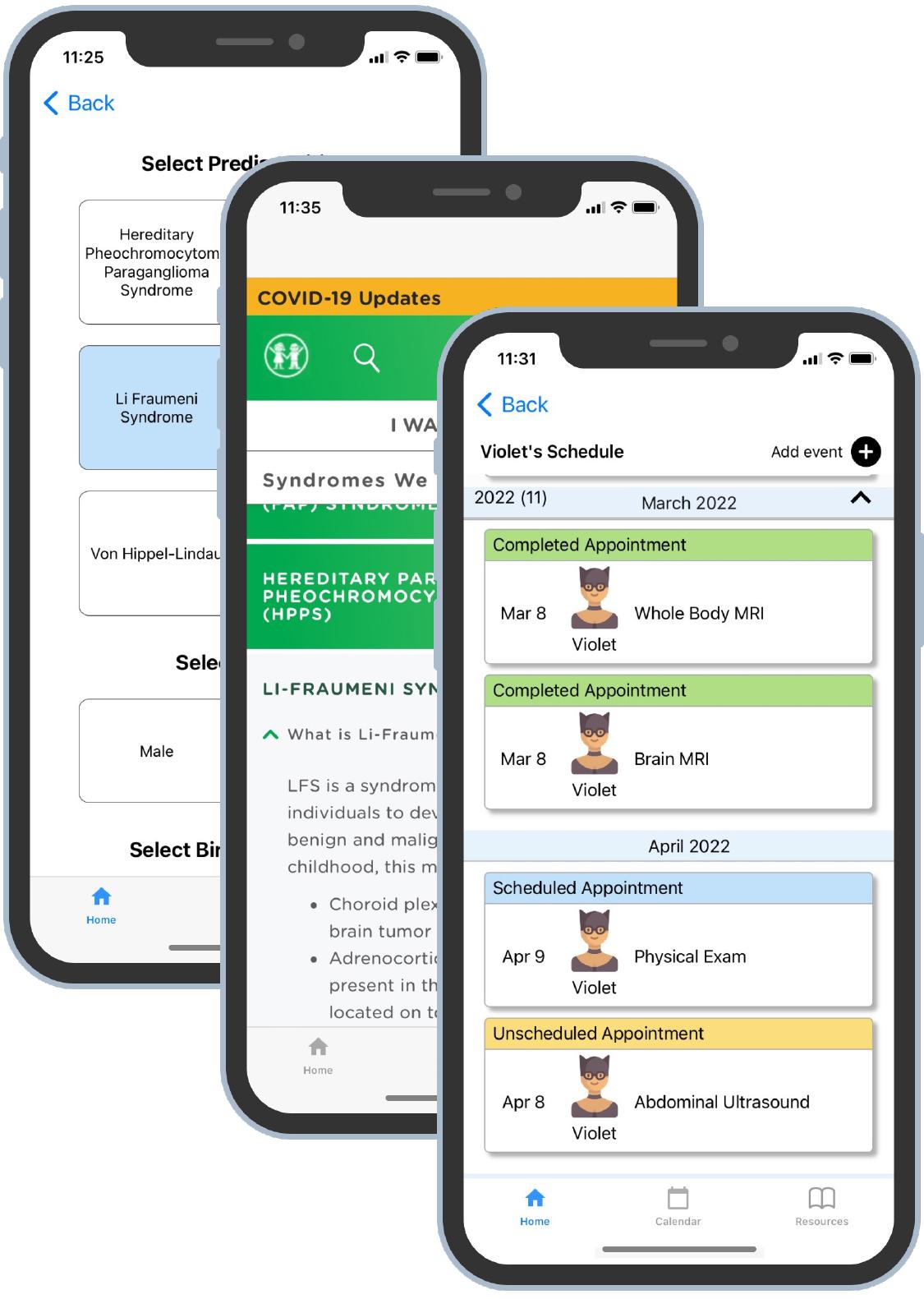
The HomeTown app helps families with cancer predispositions track their regimen of preventative tests and doctor visits.
(text and background only visible when logged in)
(text and background only visible when logged in)
When Nixon declared war on cancer back in ‘71, it may well have been wishful thinking that scientists would find a cure at all, let alone in a decade.
Susan Thomas argued “cure” isn’t really the goal anyway. She suggested the word “manage” would be more apt — and the tools Georgia Tech engineers are developing will help in that quest.
“In an aging society, you’re going to have a higher prevalence of cancer, not a lower prevalence. What we need to get to is something more like a hip implant: We have tools and we understand how to use them to prolong a healthy and rewarding life for patients.”
(text and background only visible when logged in)
(text and background only visible when logged in)
Related Stories
Three Tech Projects Tackle Cancer With ARPA-H Support
Biomedical engineers Gabe Kwong and Phil Santangelo are among the PIs on cancer detection and treatment projects.
After Cancer Battle, Raheem Beyah Has a Message
Black men are twice as likely to die from prostate cancer. Georgia Tech’s engineering dean beat it a year ago and wants more men paying attention.
Bulletin Board Material
A scrap of paper changed the life of two-time cancer survivor Josh Vose, leading him away from the operating room and into the field of medical devices.
(text and background only visible when logged in)

Helluva Engineer
This story originally appeared in the Fall 2023 issue of Helluva Engineer magazine.
With the top biomedical engineering program in the country, it’s only fitting that our rebooted College magazine focuses on health and medicine in our return issue. From cancer to anemia, synthetic biology to eye disease, Georgia Tech engineers are improving medicine today and designing a healthier tomorrow for us all.





