Researchers in the College of Engineering create new therapies to fight cancer

The numbers tell a sad story. Nearly one out of three people in the United States will have cancer during their lifetimes, according to the American Cancer Society. While a cure remains at large, innovative treatments like immunotherapies, stem cell replacement and gene therapy are advancing quickly. Screening tests are also playing a role in catching cancer early, so doctors can apply aggressive treatment to send cancer into remission.
Among those working to change the story on cancer are engineers from across Georgia Tech. It’s not just biomedical engineers, but also mechanical and electrical who are well-versed in biomechanics, diagnostic imaging, microfluidics, sensors, systems design, molecular engineering and personalized care algorithms. While these terms might not conjure up images of healthcare, medicine or clinics, they are instrumental in the identification and treatment of cancer.
Meet of a few of these engineers working to crack cancer’s code.
Catching Cancer on a Chip
Fatih Sarioglu – Assistant Professor, School of Electrical and Computer Engineering
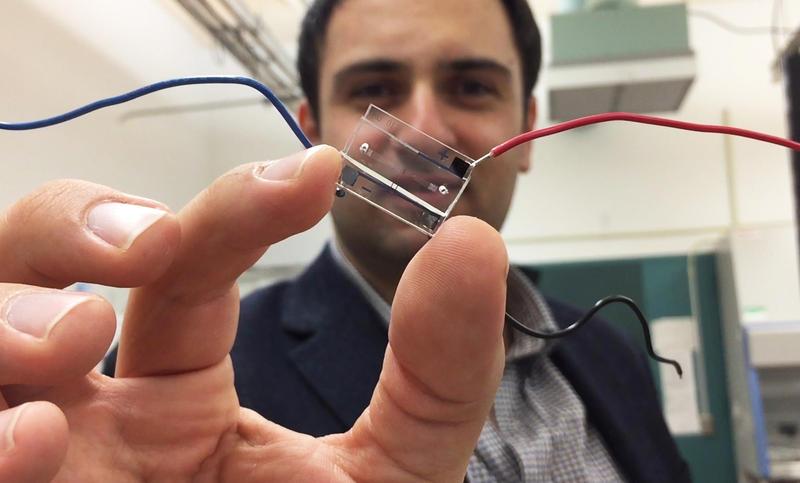
Trained as an electrical engineer, Fatih Sarioglu studies how microsystems, sensors and computational analysis can fight cancer. His Biomedical Microsystems Laboratory at Georgia Tech is creating smart chips that can diagnose and monitor cancer through blood analysis.
“When faced with the problem of cancer and the way it spreads and metastasizes, it made me consider how it is an engineering problem as much as it is a biological one,” said Sarioglu. “Cancer has many faces – it’s very heterogenous. And we need new approaches to detect it.”
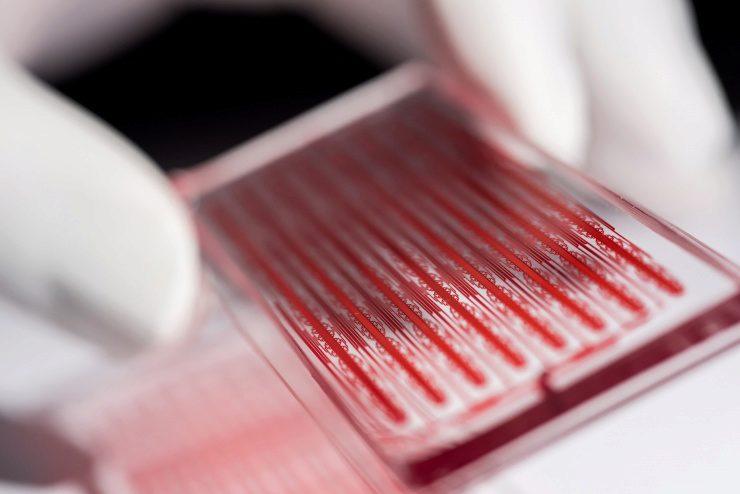
In the past, scientists would search for cancer cells in the blood using a microscope, much like searching for a needle in a haystack. The chips that Sarioglu has created are designed to literally catch cancer cells with its bifurcated design (see image below). The fluidic nature of the chip separates cancer cells from healthy blood cells as the blood flows through, enabling researchers to study these cells to discover chemical or physical markers to identify them from other cells in the body.
“The blood stream is a hostile environment, and cancer cells traveling in clusters can protect themselves,” said Sarioglu. “Because of the bifurcation, the chip catches metastasizing clustered tumor cells, while the single healthy cells keep traveling through.”
Once the chip catches the cancer cells, they can be extracted while still alive and intact and studied for metastases and gene sequences. With access to whole, undamaged cancer cells, researchers can test various treatments to determine which will have the greatest impact on the cancer outside the patient body.
Alternatively, a mutation can be detected, which can help guide the selection of the appropriate gene therapies for the patient. Catching cancer cells in the blood at this early stage may also serve as early detection before the cancer has proliferated and become symptomatic.
Massachusetts General has already licensed Sarioglu’s chip technology, and it is being used in clinical cancer studies and basic cancer research. The chip has worked on patient samples, identifying cancer cells in breast cancer, prostate cancer and melanoma.
Susan Thomas – Associate Professor, School of Mechanical Engineering
Leveraging Lymph Nodes for Immunotherapies
Susan Thomas – Associate Professor, School of Mechanical Engineering
Immunoengineering is an emerging research area that applies engineering principles for the study of the immune system. Its goal is to develop better therapies that leverage the immune system to treat disease. In Susan Thomas’ lab, immunoengineering is being applied to the lymphatic system to help the body fight cancer. Also known as cancer immunotherapy, this type of treatment leverages the body’s immune system to help patients fight and stave off disease.
“Rather than looking to chemo or radiation to fight cancer, we are now in the era of immunotherapy,” said Thomas. “Many new cancer immunotherapy drugs have been developed or are being developed and they are incredibly powerful. However, they only work in a fraction of patients. Our goal is to make these powerful cancer-fighting drugs work effectively for more patients.”
To do this, Thomas is exploring how to better deliver drugs to the lymphatic system – tissues where a large percentage of a patient’s immune cells reside.
“It may sound counterintuitive, because for so long we have tried to specifically get drugs to cancer cells only,” said Thomas. “However, immunotherapies often work directly on immune cells. If we can get these drugs to immune cells more effectively, those cells then have a better chance of fighting the cancer.”
Thomas says she is often asked why an engineer would conduct cancer research.
“Cancer is a big problem in society and engineers love to solve problems,” she explained. “Turns out, there are many engineering fundamentals that can be used to help understand cancer as a disease, as well as to develop better ways to diagnose or treat a patient.”
As an example of the engineering Thomas uses in her work, she studies the mechanics of the immune system – how fluids, molecules and cells move in the body. Mechanics help her better understand how cancers progress, develop and metastasize, which helps optimize drug delivery for targeted therapies.
“Harnessing the lymphatic system for immunotherapy really is a radical but practical and rational approach,” said Thomas. “It hasn’t been explored at all clinically. Almost every week I’m meeting with clinicians who are interested in these types of cancer therapies, but they have very little understanding of how to optimize their delivery. They can see the potential impact that engineering can having on the cancer immunotherapy field because they are hungry for new tools to use to help their patients.”
While the lymphatic targeted immunotherapies are not at in clinical testing yet, there is already talk of soon commercializing these technologies. Immunotherapy in the public conscious has exploded in recent years because of the many success stories. And Thomas hopes her work can contribute to these successes and make this type of treatment a viable option for everyone.

Julie Champion – Associate Professor, School of Chemical and Biomolecular Engineering
An Antibody Bundle that Targets Cancer Cells
Julie Champion – Associate Professor, School of Chemical and Biomolecular Engineering
Antibodies are large proteins in the body that are used by the immune system to identify and neutralize foreign objects like bacteria and viruses. A new type of immunotherapy looks to use the body’s own antibodies to target and neutralize cancer cells. Currently, antibodies themselves cannot get into a live cancer cell, but Julie Champion is creating a bundle of antibodies that can get in.
Imagine the antibody bundle as a key that unlocks the cancer cell. In Champion’s lab, she is engineering antibody protein design and assembly, combining six proteins with three therapeutic cancer-killing antibodies that can be sent directly to the cancer cell – and get inside.
“Antibodies exist to fight cancer, we just need to get them into the cells,” said Champion. “If we can do that with the protein bundle, the antibodies can kill or inhibit cancer cells from growing. Also, the bundle can deliver any antibody therapy we want it to, so it will work for various treatment regimens.”
The other benefit of an immunotherapy like Champion’s protein bundle, is that it only targets cancer cells. Many cancer therapies, such as chemo and radiation, kill all the body’s cells, even the healthy ones, leaving the patient exposed to other illnesses and with a weakened immune system. The protein bundle can carry the cancer-fighting antibody specifically to cancer cells, only destroying them.
Champion is also working on a cancer vaccine. It’s not given to prevent cancer – rather, it’s used in existing cancer patients to help their bodies better recognize the disease. Often, when a patient isn’t fighting off the cancer like it should, it’s because the body doesn't’t recognize it as foreign – the cancer has tricked the immune system into thinking it belongs, therefore suppressing the usual immune response. The vaccine stimulates the immune cells and ensures the body knows the cancer cells are foreign.
The vaccine research in Champion’s lab also leverages protein design to build nanoparticles directly out of peptides identified from the surface of cancer cells. These particles train and stimulate the immune system to recognize and fight only cancer cells that have the peptide on them, leaving the healthy cells alone and intact. The cancer vaccine is also a durable cure. If the immune system can recognize and fight the cancer, when it comes back in five years, the body will know how to fight it again.
“The power of immunotherapy is that you’re working with what’s already in your body,” said Champion. “So you can engage the body to fight for itself.”
In the future, Champion is considering how she can scale up production of the protein bundles to make them a viable therapy for more patients at a lower cost. The hope is that in five to 10 years, all cancer patients will have access to cutting edge treatments, giving them more than a fighting chance.