Researchers etch nano-sized textures and add copper ions to create a naturally antibacterial material for hospitals and other shared settings.

Postdoctoral scholar Anuja Tripathi examines a small sample of stainless steel after an electrochemical etching process she designed to create nano-scale needle-like structures on its surface. A second process deposits copper ions on the surface to create a dual antibacterial material.
An electrochemical process developed at Georgia Tech could offer new protection against bacterial infections without contributing to growing antibiotic resistance.
The approach capitalizes on the natural antibacterial properties of copper and creates incredibly small needle-like structures on the surface of stainless steel to kill harmful bacteria like E. coli and Staphylococcus. It’s convenient and inexpensive, and it could reduce the need for chemicals and antibiotics in hospitals, kitchens, and other settings where surface contamination can lead to serious illness.
It also could save lives: A global study of drug-resistant infections found they directly killed 1.27 million people in 2019 and contributed to nearly 5 million other deaths — making these infections one of the leading causes of death for every age group.
Researchers described the copper-stainless steel and its effectiveness May 20 in the journal Small.
“Killing Gram-positive bacteria without chemicals is comparatively easy but tackling Gram-negative bacteria poses a significant challenge, due to their thick, multilayered cell membrane. And if these bacteria persist on surfaces, they can grow rapidly,” said Anuja Tripathi, the study’s lead author and a postdoctoral scholar in the School of Chemical and Biomolecular Engineering. “I aimed to develop an antibiotic-free bactericidal surface effective against Gram-negative and Gram-positive bacteria.”
Tripathi and her colleagues — William R. McLain Professor Julie Champion and former Ph.D. students Jaeyoung Park and Thomas Pho — produced a one-two punch that overcomes those challenges and doesn’t help bacteria develop resistance to drugs.
The team first developed an electrochemical method to etch the surface of stainless steel, creating nano-sized needle-like structures on the surface that can puncture bacteria’s cell membranes. Then, with a second electrochemical process, the researchers deposited copper ions on the steel’s surface.
Copper interacts with the cell membranes and ultimately compromises them.
“The nanotextured stainless steel can kill both Gram-negative and Gram-positive bacteria, but we wanted to enhance the antibacterial activity for surfaces that can be highly contaminated,” Tripathi said. “The copper coating on the nanotextured stainless steel gave us very high antibacterial activity.”
(text and background only visible when logged in)

During the Tripathi's electrochemical process, current and an acid electrolyte etch nano-sized needle-like structures on the surface of stainless steel. The structures are able to destroy bacterial cells.

These four samples of stainless steel show the different stages of Tripathi's process. At left, an unmodified sample at the top and a sample after the electrochemical etching process at the bottom. On the right, two samples after copper ion deposition — four minutes for the top piece and 15 minutes for the bottom piece.
Despite copper’s known antibacterial properties, it’s not widely used to fight surface contamination because it’s expensive. Tripathi’s approach deposits only a thin layer of copper ions on the stainless steel, so it’s cost-effective without compromising the material’s antibacterial activity.
Together, the dual attacks resulted in 97% reduction of Gram-negative E. coli and 99% reduction in Gram-positive Staphylococcus epidermis bacteria in the group’s study.
Tripathi said the stainless steel could be used for common tools in medical settings that are easily fouled, such as scissors or tweezers. It could be used for door handles, stair railings, and perhaps even sinks — places where stainless steel is often the material of choice and surface bacteria are common, especially in hospitals or other shared settings.
The process she and her colleagues developed also could be useful in food service. Tripathi said the approach could be fairly easily incorporated into existing industrial processes, where different electrochemical coating methods already are used for stainless steel food storage containers.
Tripathi said future work will investigate if the copper-coated, nanotextured stainless steel is effective against other kinds of cells harmful to human health. She’s also interested in exploring whether the steel could be used for medical implants to help ward off infections.
Since it proved effective against troublesome E. coli, she’s hopeful.
“Reflecting on a recent E. coli outbreak in grocery stores in Calgary, Canada, I was particularly driven in my research, recognizing the urgent relevance and significance of combating such resilient bacteria on surfaces,” Tripathi said. “They can be difficult to eliminate. So, if we can effectively eliminate E. coli, we stand a good chance of eradicating many bacteria on surfaces.”
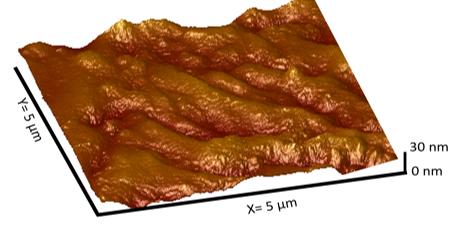
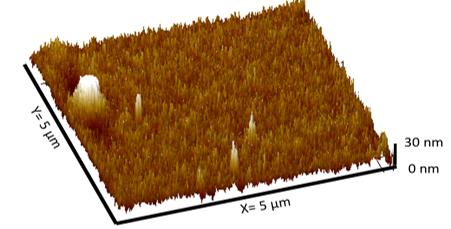
Atomic force microscope images show a stainless steel sample before electrochemical etching (top) and after the process (bottom). Tripathi's process creates needle-like protrusions at the nanometer scale that are capable of killing bacteria cells. (Images Courtesy: Anuja Tripathi)
About the Research
This research was supported by the Presidential Postdoctoral Fellowship at Georgia Tech and performed in part at the Georgia Tech Institute for Electronics and Nanotechnology, a member of the National Nanotechnology Coordinated Infrastructure supported by the National Science Foundation, grant No. ECCS-2025462. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of any funding agency.
Citation: A. Tripathi, J. Park, T. Pho, J. A. Champion, Dual Antibacterial Properties of Copper-Coated Nanotextured Stainless Steel. Small 2024, 2311546. https://doi.org/10.1002/smll.202311546

Preeminence in Research
Related Content

New Approach Could Make Reusing Captured Carbon Far Cheaper, Less Energy-Intensive
A team led by Marta Hatzell designed a new electrochemical reactor to seamlessly integrate into direct air capture systems and turn CO2 into useful raw materials.

ChBE, Meta Researchers Create Massive Open Dataset to Advance AI Solutions for Carbon Capture
The project aims to accelerate direct air capture development while significantly reducing costs by giving engineers a jumpstart on potential materials for any environmental conditions.